Gold decoration of silica by decomposition of aqueous gold(iii) hydroxide at low temperatures†
Abstract
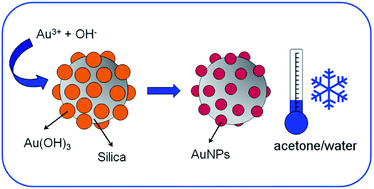
The decomposition of gold hydroxide to give metallic gold is known to take place around 300 °C in dry environments. However, little information about the gold hydroxide stability in wet environments has been recorded. Here, we present experimental evidence which shows that aqueous/water-enriched gold(III) hydroxide colloids decompose spontaneously to form gold nanoparticles at temperature values above the freezing point of water. Based on this reaction, we developed a method to decorate silica spheres with gold nanoparticles by precipitation and decomposition of gold(III) hydroxide onto the silica surface in wet media by a simple one-pot/one-step protocol. The silica|gold nanostructures are prepared in high yield and with a low level of by-products.


 Please wait while we load your content...
Please wait while we load your content...