Enzymatic synthesis of nucleoside analogues from uridines and vinyl esters in a continuous-flow microreactor†
Abstract
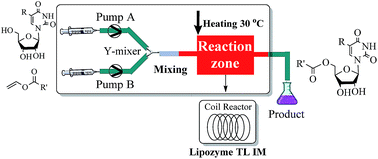
We achieved the effective controllable regioselective acylation of the primary hydroxyl group of uridine derivatives catalyzed by Lipase TL IM from Thermomyces lanuginosus with excellent conversion and regioselectivity. Various reaction parameters were studied. These regioselective acylations performed in continuous flow microreactors are a proof-of-concept opening the use of enzymatic microreactors in uridine derivative biotransformations.


 Please wait while we load your content...
Please wait while we load your content...