Metabolism of five diterpenoid lactones from Dioscorea bulbifera tubers in zebrafish†
Abstract
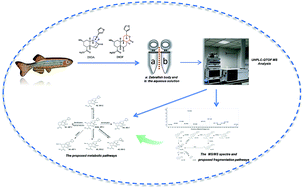
Diterpenoid lactones (DLs) have been reported to be the main hepatotoxic constituents in Dioscorea bulbifera tubers (DBT), a traditional Chinese medicinal herb. The acquisition of early information regarding its metabolism is critical for evaluating the potential hepatotoxicity of DLs. We investigated, for the first time, the main metabolites of diosbulbin A (DIOA), diosbulbin C (DIOC), diosbulbin (DIOG), diosbulbin (DIOM) and diosbulbin (DIOF) in adult zebrafish. By using ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC-QTOF MS), 6, 2, 7, 5 and 4 metabolites of DIOA, DIOC, DIOF, DIOM and DIOG were identified in the zebrafish body and the aqueous solution, respectively. Both phase-I and phase-II metabolites were observed in the metabolic profiles and the metabolic pathways involved in hydroxyl reduction, glucuronidation, glutathione conjugation and sulfation. The above results indicated that hepatocytic metabolism might be the major route of clearance for DLs. This study provided important information for the understanding of the metabolism of DLs in DBT.


 Please wait while we load your content...
Please wait while we load your content...