Hydration of the methanesulfonate–ammonia/amine complex and its atmospheric implications†
Abstract
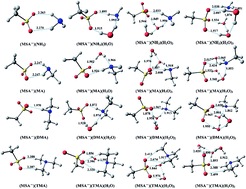
Methanesulfonate (MSA−), found in substantial concentrations in the atmosphere, is expected to enhance aerosol nucleation and the growth of nanoparticles, but the details of methanesulfonate clusters are poorly understood. In this study, MSA− was chosen along with ammonia (NH3) or three common amines and water (H2O) to discuss the roles of ternary homogeneous nucleation and ion-induced nucleation in aerosol formation. We studied the structural characteristics and thermodynamics of the clusters using density functional theory at the PW91PW91/6-311++G(3df,3pd) level. The analysis of noncovalent interactions predicts that the amines can form more stable clusters with MSA− than NH3, in agreement with the results from structures and thermodynamics; however, the enhancement in stability for amines is not large enough to overcome the difference in the concentrations of NH3 and amines under typical atmospheric conditions. In addition, the favorable free energies of formation for the (MSA−)(NH3/amines)(H2O)n (n = 0–3) clusters at 298.15 K show that MSA− could contribute to the aerosol nucleation process with binding NH3/amines and H2O up to n = 3. There are strong temperature and humidity dependences for the formation of complexes; higher humidity and temperature promote the formation of larger hydrates. Finally, for the (MSA−)(NH3/amines)(H2O)n clusters, the evaporation rates were determined to further investigate the atmospheric implications.


 Please wait while we load your content...
Please wait while we load your content...