A high energy density asymmetric supercapacitor utilizing a nickel phosphate/graphene foam composite as the cathode and carbonized iron cations adsorbed onto polyaniline as the anode†
Abstract
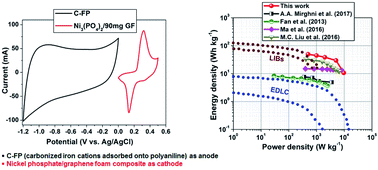
This work presents the effect of different contents of graphene foam (GF) on the electrochemical capacitance of nickel phosphate Ni3(PO4)2 nano-rods as an electrode material for hybrid electrochemical energy storage device applications. Pristine Ni3(PO4)2 nano-rods and Ni3(PO4)2/GF composites with different GF mass loadings of 30, 60, 90 and 120 mg were synthesised via a hydrothermal method. The electrochemical behavior of pristine Ni3(PO4)2 and Ni3(PO4)2/GF composites were analysed in a three-electrode cell configuration using 6 M KOH electrolyte. The Ni3(PO4)2/90 mg GF composite sample exhibited the highest specific capacity of 48 mA h g−1 at a current density of 0.5 A g−1. The electrochemical behavior of the Ni3(PO4)2/90 mg GF composite was further analysed in a two-electrode hybrid asymmetric device. A hybrid asymmetric device was fabricated with Ni3(PO4)2/90 mg GF as the cathode and carbonized iron cations (Fe3+) adsorbed onto polyaniline (PANI) (C-FP) as the anode material (Ni3(PO4)2/90 mg GF//C-FP) and tested in a wide potential window range of 0.0–1.6 V using 6 M KOH. This hybrid device achieved maximum energy and power densities of 49 W h kg−1 and 499 W kg−1, respectively, at 0.5 A g−1 and had long-term cycling stability.


 Please wait while we load your content...
Please wait while we load your content...