A simple hydrazone as a multianalyte (Cu2+, Al3+, Zn2+) sensor at different pH values and the resultant Al3+ complex as a sensor for F−†
Abstract
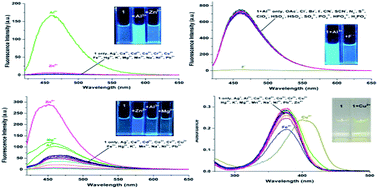
A new colorimetric and fluorescence molecular chemosensor based on triazole hydrazone can be used as a multi-probe for selective detection of Al3+, Zn2+, and Cu2+ by monitoring changes in the absorption and fluorescence spectral patterns. Results show that Al3+ and Zn2+ ions can induce remarkable fluorescence enhancement at pH 6.0 and pH 10.0, respectively, while the addition of Cu2+ ions leads to a significant UV-visible absorption enhancement in the visible range at pH 6.0. In addition, the resultant Al3+ complex could act as an ‘on–off’ fluorescence sensor for F−. The fluorescence sensor was also used to monitor intracellular Al3+, Zn2+, and F− in Hela cells.


 Please wait while we load your content...
Please wait while we load your content...