Effect of C60 on the phase transition behavior of a lipid bilayer under high pressure
Abstract
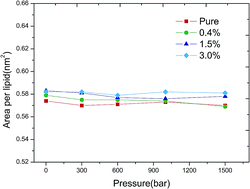
Interactions between fullerenes and cells and effects on the main transition of lipid bilayers have attracted much attention in biophysics in recent years. By employing coarse-grained molecular dynamics simulations, we obtained the temperature–pressure phase diagrams of a dipalmitoylphosphatidylcholine bilayer, which exhibits a gel phase and a fluid phase, with variation of the C60 versus lipid ratios. The simulation results show that the critical area per lipid at the fluid–gel main phase transition boundary increases with the increasing ratios of C60. A critical area per lipid of 0.594 ± 0.002 nm2 is obtained when the ratio of C60 reaches 6.4% while that of the pure bilayer case is 0.572 ± 0.002 nm2. The main transition temperature, Tm, remains almost unchanged with the addition of C60 below a ratio of 4.7%, while a 2 K decrease of Tm is observed at a ratio of 6.4% under various pressures. Consequently, the presence of C60 in the bilayer, with the ratio of C60 less than 4.7%, will not influence the main transition behavior of the bilayer even under pressure as high as 1500 bar. The radial distribution function analyses suggest that the presence of C60 produces no impact on the radial distribution of the lipids in the bilayers. The lateral density profiles show that the incorporation of C60 with relatively high ratios stabilizes the thickness of the bilayer.


 Please wait while we load your content...
Please wait while we load your content...