Characterization of protein adsorption on stretched polyurethane nanofibers prepared by electrospinning
Abstract
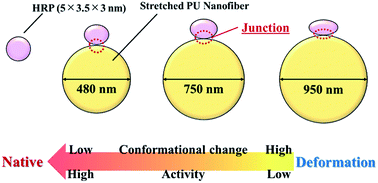
Conformation and activity control of proteins adsorbed on certain material surfaces enables the development of numerous high-performance applications. Herein, we examined the relationship between the diameter (surface shape) of polyurethane (PU) nanofibers and the conformation/activity of proteins adsorbed thereon, showing that hard segments align linearly in the long-axis direction when the PU structure is changed from random-segment to stretched nanofibers. Moreover, we revealed that the clustering of hydrophobic hard PU segments and protein adsorption are caused by hydrophobic interactions. Proteins adsorbed on thick nanofibers (diameter = 950 nm) showed decreased activity due to large conformational changes, whereas those adsorbed on thin nanofibers (diameter = 480 nm) retained a close-to-natural shape and thus showed relatively high activity, confirming that the shape of PU nanofiber surface affects the conformation and activity of proteins adsorbed thereon.


 Please wait while we load your content...
Please wait while we load your content...