Asymmetric synthesis of 2,3-disubstituted indolines via an organocatalytic intramolecular Michael addition†
Abstract
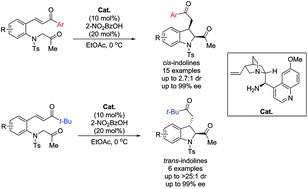
An asymmetric synthesis of 2,3-disubstituted indolines has been developed via an organocatalytic intramolecular Michael addition. When a primary amine derived from cinchona alkaloid was used as the catalyst, the intramolecular cyclization reaction of (E)-3-(2-(2-oxopropylamino)aryl)-1-arylprop-2-en-1-ones afforded the corresponding cis-2,3-disubstituted indoline derivatives with high yields, moderate diastereoselectivities, and excellent enantioselectivities (up to 2.7 : 1 dr and 99% ee). Moreover, the catalytic reaction of (E)-3-(2-(2-oxopropylamino)aryl)-1-alkylprop-2-en-1-ones afforded trans-2,3-disubstituted indolines in high yields and with good-to-excellent diastereo- and enantioselectivities (up to 20 : 1 dr and 99% ee).


 Please wait while we load your content...
Please wait while we load your content...