Microwave-assisted reduction method under nitrogen atmosphere for synthesis and electrical conductivity improvement of reduced graphene oxide (rGO)†
Abstract
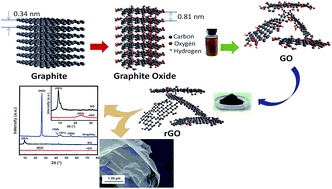
A facile method to synthesize rGO using a microwave-assisted method under N2-atmosphere conditions is reported. Two different reducing agents were used in this research, hydrazine hydrate and L-ascorbic acid (L-AA). The reduction of graphene oxide was completed in 3 minutes by the microwave-assisted method under N2-atmosphere conditions. The structures and morphologies of both rGOs prepared by this new method were confirmed by attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectrometry, X-ray diffraction (XRD), Raman spectroscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM) and energy dispersive X-ray spectrometry (EDX), confirming the formation of rGO. Four-point probe measurements showed an electrical conductivity of 18.10 S cm−1 for the rGO reduced by hydrazine hydrate and 12.02 S cm−1 for the rGO reduced by L-AA. Combining this new method with annealing at 300 °C for 30 min resulted in a higher electrical conductivity: up to 23.26 S cm−1 for the rGO reduced by hydrazine hydrate and up to 16.19 S cm−1 for the rGO reduced by L-AA. This study provides a new perspective on synthesizing rGO with high electrical conductivity using an effective and efficient method.


 Please wait while we load your content...
Please wait while we load your content...