One-pot, two-step synthesis and photophysical properties of 2-(5-phenylindol-3-yl)benzimidazole derivatives†
Abstract
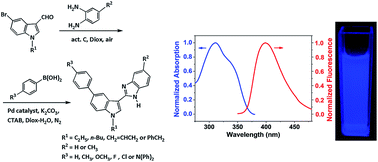
An efficient one-pot, two-step procedure has been successfully applied for the synthesis of a series of 2-(5-phenylindol-3-yl)benzimidazoles. The first step was cyclocondensation–oxidation of 5-bromoindole-3-aldehydes with o-phenylenediamines in 1,4-dioxane, which was promoted by activated carbon and used atmospheric air as a “green” oxidant. The resulting 2-(5-bromoindol-3-yl)benzimidazoles in the pot were in situ coupled with postadded phenylboronic acids, and catalyzed with PdCl2(dppf) in 1,4-dioxane-H2O to afford the desired compounds with satisfactorily high yields. The relationship between the synthesized compounds' absorption, and fluorescence spectra with molecular structures has been investigated with experimental data and theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...