Enhanced adsorption capacity and selectivity towards strontium ions in aqueous systems by sulfonation of CO2 derived porous carbon†
Abstract
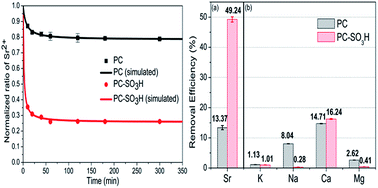
Oxygen-enriched carbon materials derived from carbon dioxide were functionalized using sulfonic acid to remove Sr2+ ions from aqueous solutions. Synthesized sulfonated porous carbon materials (PC-SO3H) showed higher adsorption capacity and selectivity towards Sr2+ than non-functionalized porous carbons (PC). The formation of the C-SO3H functional group in PC-SO3H and its ability to proton exchange with Sr2+ was the main contributor to the enhanced performance. The maximum uptake capacity of Sr2+ by PC-SO3H was 18.97 mg g−1, which was 1.74 times greater than PC. PC-SO3H removed 99.9% and 97.6% of Sr2+ from aqueous solutions with initial Sr2+ concentrations of 5 mg L−1 and 10 mg L−1, respectively. Sr2+ adsorption showed rapid kinetics, reaching the adsorption equilibrium within 1 h with high adsorption capacity at equilibrium which is 3.52 times greater than that of PC. Additionally, PC-SO3H selectively adsorbed Sr2+ even in the presence of excess amounts of competing ions. Sulfonation of oxygen-enriched carbon had a significant effect on enhancing the affinity towards Sr2+ and suppressing adsorption towards other competing ions.


 Please wait while we load your content...
Please wait while we load your content...