Unveiling orbital coupling at the CoPc/Bi(111) surface by ab initio calculations and photoemission spectroscopy
Abstract
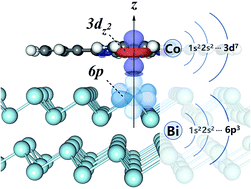
The interfacial electronic structures of cobalt phthalocyanine adsorbed on a semimetal Bi(111) surface have been systematically investigated herein. Our study first indicates that the CoPc molecule is quite sensitive to the adsorption site on the relatively inert Bi(111) surface. Secondly, apparent change of the electronic structures of CoPc has been revealed upon adsorption as compared to that in the gas phase, due to the orbital coupling between the cobalt partial empty state and the surface state from the bismuth substrate and interfacial charge transfer. Interestingly, the local magnetic moment is still retained for the adsorbed CoPc molecule on the diamagnetic Bi(111) surface, which is different to that on other noble metal substrates. Analysis of the charge density difference and the Bader charge provides evident insight on the mechanism of interfacial charge transfer which is chiefly mediated by the central Co atom and the weak vdW dispersion between the π-conjugated macrocyclic ligand and the bismuth substrate, as further confirmed by experimental XPS results. In the end, our report may provide an appealing route towards the fundamental understanding and reliable engineering of interfacial interactions between magnetic and semimetal nanostructures.


 Please wait while we load your content...
Please wait while we load your content...