Theoretical study of the Cl-initiated atmospheric oxidation of methyl isopropenyl ketone†
Abstract
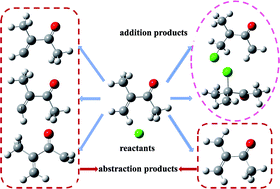
The Cl-initiated atmospheric oxidation mechanism of methyl isopropenyl ketone (MIK) has been investigated at the CCSD(T)/6-311++G(d,p)//MP2/6-311G(d,p) level of theory. Two reaction types initiated from Cl-addition and H-abstraction, respectively, and the key intermediates involved, IM1, IM2 (obtained from Cl-addition) and IM6 (obtained from H-abstraction), are presented and discussed. The calculated results supported the experimental results that Cl addition dominates the initial reactions of MIK with Cl atoms, and the most energetically favorable pathway is the Cl addition to the terminal carbon of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. Among the four proposed H abstraction processes, our study clearly indicated that the H-abstraction by Cl only takes place at the methyl linking to the internal alkenfinic carbon rather than the one at the methyl linking to the carbonyl carbon, which resolves the uncertainty of H-abstraction encountered in experiment. In addition, the isomerization processes involved in the Cl addition mechanism (1,4-H shift isomerization of IMa3 and 1,5-H shift isomerization of IMb3) were proposed in this work and found to be feasible. Both the major products experimentally detected and those derived from our theoretical study have been identified. The rate constants of the initial reactions over the atmospheric temperature range of 180–380 K have been determined using the MESMER program on the basis of Rice–Ramsperger–Kassel–Marcus (RRKM) theory. One the basis of the kinetic data we obtained, the Arrhenius formula of the total rate constant has been deduced: ktot = (5.31 × 10−11)exp(−335.24/T) cm3 per molecule per s. The atmospheric lifetime of MIK in the presence of Cl atoms is calculated to be about 170.01 h.
C bond. Among the four proposed H abstraction processes, our study clearly indicated that the H-abstraction by Cl only takes place at the methyl linking to the internal alkenfinic carbon rather than the one at the methyl linking to the carbonyl carbon, which resolves the uncertainty of H-abstraction encountered in experiment. In addition, the isomerization processes involved in the Cl addition mechanism (1,4-H shift isomerization of IMa3 and 1,5-H shift isomerization of IMb3) were proposed in this work and found to be feasible. Both the major products experimentally detected and those derived from our theoretical study have been identified. The rate constants of the initial reactions over the atmospheric temperature range of 180–380 K have been determined using the MESMER program on the basis of Rice–Ramsperger–Kassel–Marcus (RRKM) theory. One the basis of the kinetic data we obtained, the Arrhenius formula of the total rate constant has been deduced: ktot = (5.31 × 10−11)exp(−335.24/T) cm3 per molecule per s. The atmospheric lifetime of MIK in the presence of Cl atoms is calculated to be about 170.01 h.


 Please wait while we load your content...
Please wait while we load your content...