Synthesis of 1,2-disubstituted benzimidazoles using an aza-Wittig-equivalent process†
Abstract
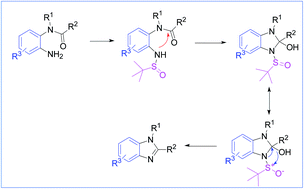
A synthetic approach for 1,2-disubstituted benzimidazoles has been successfully designed based on effective C–N bond construction, which demonstrated mild reaction conditions and excellent yields. The method involves treating derivatives of o-phenylenediamine with tert-butanesulfoxide and NBS under acidic conditions, which undergoes an aza-Wittig-equivalent process to afford the desired products. Using this method, a series of benzimidazoles containing multiple functional groups with varying electronic effects have been successfully constructed.


 Please wait while we load your content...
Please wait while we load your content...