Insight into the excited-state double proton transfer mechanisms of doxorubicin in acetonitrile solvent†
Abstract
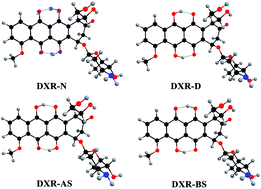
Doxorubicin (DXR), having double intramolecular hydrogen bonds, is theoretically investigated with an aim to explore the excited-state intramolecular double proton transfer (ESIDPT) mechanism regarding stepwise versus synchronous double proton transfer. The changes in the calculated primary bond lengths and bond angles testify that the intramolecular hydrogen bonds (O1–H2⋯O3 and O4–H5⋯O6) are both strengthened in the S1 state (ca. the S0 state), which provides the possibility for the ESIDPT reaction. We adopt the TDDFT and CIS methods coupled with transition state (TS) theory to further elucidate the ESIDPT mechanism. The potential energy surfaces (PESs) are successfully constructed using different basis sets. Three kinds of stable structure are found on the S1 PES. Herein, we establish a new elaborated ESIDPT mechanism for DXR. This new mechanism unambiguously excludes synchronous double proton transfer, while the stepwise double proton transfer mechanism is theoretically confirmed. This systematic investigation into the photophysical properties of the antineoplastic drug DXR may be significant in obtaining fundamental insight into the transport mechanism of DXR inside cultured cells.


 Please wait while we load your content...
Please wait while we load your content...