Synthesis, structures and magnetic properties of isoreticular polyrotaxane-type two-dimensional coordination polymers†
Abstract
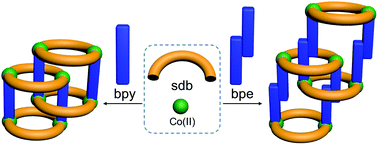
Two isoreticular polyrotaxane-type two-dimensional coordination polymers (2D CPs) [Co2(bpy)(sdb)2]·2H2O (1) and [Co2(bpe)(sdb)2]·DMA·1.5H2O (2) (where sdb = 4,4′-sulfonyldibenzoate; bpy = 4,4′-bipyridyl and bpe = 4,4′-bipyridyl ethylene) have been synthesized and their solid state structures have been determined by X-ray crystallography. The solid state structures made up of wheels and axles have been synthesized using a bent dicarboxylate (sdb), two linear spacer ligands and Co(II). Interestingly, the sheets are two-fold entangled by [Co2(sdb)2Co2] wheels and bpy or bpe axles. Due to the shorter length of the bpy ligand, the [Co2(sdb)4] moieties in 1 are brought closer than in the sheet formed in 2. The magnetic properties of 1 and 2 have been discussed and fitted according to a spin Hamiltonian. 1 showed predominantly antiferromagnetic interactions with exchange integral of −1.522 cm−1. While 2 exhibited the paramagnetic behaviour below 40 K and antiferromagnetic behaviour at higher temperature.


 Please wait while we load your content...
Please wait while we load your content...