Internal asymmetric induction by the C-6 substituent on the oxidation reaction of interglycosidic sulfur atom of thiodisaccharides†
Abstract
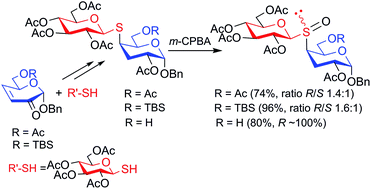
Since the enantio or diastereoselective preparation of sulfoxides is a current challenge, we explore the possibility of inducing diastereoselectivity in the oxidation of the sulfur atom of thiodisaccharides, according to their substitution patterns. Thus, a series of 3-deoxy-4-S-(β-D-glucopyranosyl)-4-thio-β-D-xylo-hexopyranoside derivatives, with different substituents at C-6 (OH, OAc or OTBS) of the reducing end, have been synthesized and treated with m-CPBA for the oxidation of the sulfur atom at C-4, which is vicinal to C-6. The absolute configuration at the sulfur stereocenter of the resulting sulfoxides was established taking into account shielding/deshielding anisotropic effects of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond on the chemical shift of the NMR signals of selected protons, in the most populated syn ϕ/syn ψ conformation of the thiodisaccharide S-oxides. The OAc and OTBS derivatives afforded diastereomeric mixtures of R and S sulfoxides in a similar ratio (1.4 : 1 and 1.6 : 1, respectively). In contrast, the oxidation of thiodisaccharide with a free hydroxyl group at C-6 was completely diastereoselective in favor of the R sulfoxide. The influence of the thiodisaccharide C-6 substituent on the stereochemical course of the oxidation is discussed.
O bond on the chemical shift of the NMR signals of selected protons, in the most populated syn ϕ/syn ψ conformation of the thiodisaccharide S-oxides. The OAc and OTBS derivatives afforded diastereomeric mixtures of R and S sulfoxides in a similar ratio (1.4 : 1 and 1.6 : 1, respectively). In contrast, the oxidation of thiodisaccharide with a free hydroxyl group at C-6 was completely diastereoselective in favor of the R sulfoxide. The influence of the thiodisaccharide C-6 substituent on the stereochemical course of the oxidation is discussed.


 Please wait while we load your content...
Please wait while we load your content...