Photo-sensitive benzoxazine II: chalcone-containing benzoxazine and its photo and thermal-cured thermoset†
Abstract
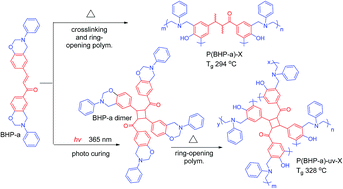
A chalcone-containing benzoxazine (BHP-a) was synthesized from a chalcone-containing bisphenol: 1,3-bis(4-hydroxyphenyl) propanone (BHP), aniline and paraformaldehyde in a co-solvent of xylene/1-butanol (2/1, V/V). The structure of BHP-a was successfully confirmed by FTIR, 1H and 13C-NMR spectra. After thermal treatment at a temperature higher than 240 °C, BHP becomes insoluble. This indicates that the double bond of the chalcone moiety of BHP can be thermally polymerized at elevated temperature. The UV spectrum shows that the chalcone moiety of BHP-a underwent dimerization via [2π + 2π] cycloaddition. Therefore, two procedures were applied to cure BHP-a. The first one was thermal curing of chalcone and oxazine moieties of BHP-a. The second one was photo curing of the chalcone moiety, followed by thermal curing of the oxazine moiety. The thermal properties of thermosets based on the two procedures were evaluated. Thermosets of BHP-a exhibit a Tg as high as 294 °C for curing procedure one, and 328 °C for curing procedure two. The value is much higher than that of a traditional bisphenol/aniline-based benzoxazine thermoset. We conclude that the curing of the double bond of the chalcone and photo dimerization of the chalcone contribute to the good thermal properties.


 Please wait while we load your content...
Please wait while we load your content...