Hormesis of some organic solvents on Vibrio qinghaiensis sp.-Q67 from first binding to the β subunit of luciferase†
Abstract
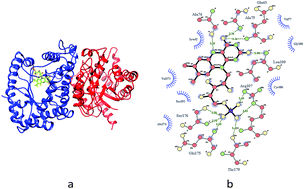
Hormesis is a biphasic concentration–response relationship. During the luminescence inhibition test of Vibrio qinghaiensis sp.-Q67 (Q67), some organic solvents display the hormesis phenomenon. However, the mechanism of hormesis with respect to organic solvents remains unclear. This study focuses on luciferase, which is the key factor in luminescent reactions, and explores its role in the mechanism of hormesis. Q67 luciferase has two subunits, α and β. Molecular docking and molecular dynamics simulations were carried out by taking organic solvents as ligand and the two subunits as receptor. In addition, the binding free energies of the complexes formed by the ligand and Q67 luciferase were calculated. The results showed that the organic solvent ligands exhibiting hormesis bind to the β subunit first, while those that do not exhibit hormesis bind more easily to the α subunit. The hormetic organic solvents bind to β subunit first at low concentration, and change the flexibility of residues Ser145–Arg165 located on the α subunit; this enables flavin mononucleotide (FMN) to bind to the α subunit, exhibiting the hormesis phenomenon. With the increasing concentration, redundant molecules start to bind to the α subunit and compete with/block FMN binding to the α subunit, resulting in inhibition.


 Please wait while we load your content...
Please wait while we load your content...