Mono and double Mizoroki–Heck reaction of aryl halides with dialkyl vinylphosphonates using a reusable palladium catalyst under aqueous medium†
Abstract
Operationally-simple and reusable Pd-catalyzed mono and double Mizoroki–Heck reactions of aryl halides and dialkyl vinylphosphonates using iPr2NH as a base in aqueous medium under air were developed. For aryl iodides, the reaction could be conducted at 80 °C under a low catalyst loading (0.1–1 mol%). When aryl bromides were applied, however, a greater amount of catalyst (5 mol%) and a longer reaction time at 120 °C were required. Simply changing dialkyl vinylphosphonates as the limiting reagent led to the formation of 2,2-diaryl vinylphosphonates in good to high yields. After reaction, the residual aqueous solution could be reused for both mono and double Mizoroki–Heck reactions, making the reactions greener and reducing wastage of precious metals and use of harmful organic solvents as the reaction medium.
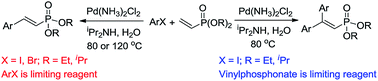


 Please wait while we load your content...
Please wait while we load your content...