Synthesis and biological evaluation of novel neoflavonoid derivatives as potential antidiabetic agents†
Abstract
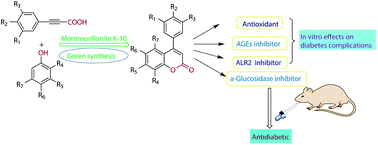
Various substituted neoflavonoid derivatives were synthesized using sulfated montmorillonite K-10 as a catalyst. This method is environmental friendly, sustainable and economical, convenient in isolation and purification processes, with little byproducts, using earth-abundant catalysts and has relatively high yield. Those neoflavonoid derivatives were screened for antioxidant, a-glucosidase inhibitory, aldose reductase 2 (ALR2) inhibitory and advanced glycation end-product formation inhibitory effects. Most compounds exhibited significant antioxidant and advanced glycation end-product (AGE) formation inhibitory activities. It was interesting to note that out of thirty compounds, 8k and 8l were found to have greater ALR2 inhibitory activity than the standard drug quercetin. The pharmacological studies suggested neoflavonoid with adjacent 7,8-dihydroxy groups were more effective in inhibiting ALR2. Antidiabetic activity studies had shown that compounds 8l and 8m were equipotent to the standard drug glibenclamide in vivo. In summary, the target compound 8l provided a potential drug design concept for the development of therapeutic or prophylactic agents of diabetes and diabetes complications.


 Please wait while we load your content...
Please wait while we load your content...