Cascade catalytic hydrogenation–cyclization of methyl levulinate to form γ-valerolactone over Ru nanoparticles supported on a sulfonic acid-functionalized UiO-66 catalyst†
Abstract
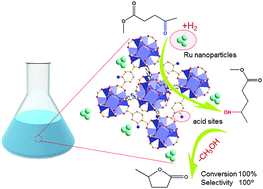
We herein report a high-yielding one-pot upgrade strategy for converting biomass-derived methyl levulinate (ML) into γ-valerolactone (GVL) over a dual-functional catalyst prepared by depositing Ru nanoparticles on a sulfonic acid-functionalized Zr-based metal–organic framework (SO3H-UiO-66). Under the mild conditions of 80 °C and 0.5 MPa H2 for 4 h in aqueous solution, a quantitative (100%) yield of GVL was obtained over the prepared Ru/SO3H-UiO-66 catalyst. In contrast, a very limited yield of GVL was achieved in the control experiment by first hydrogenating the reactant ML over a metal catalyst without any acidity (e.g. Ru/C) to produce the 4-hydroxypentanoic acid methyl ester (4-HPME) intermediate, followed by treatment of this intermediate over the acidic SO3H-UiO-66 support in the absence of metal. We also found that the catalytic activity and selectivity of Ru/SO3H-UiO-66 were significantly suppressed upon neutralization of its acidic sites, thereby confirming the indispensable role of the sulfonic acid groups in promoting the intramolecular dealcoholation of the 4-HPME intermediate. Furthermore, the Ru/SO3H-UiO-66 catalyst was recyclable over five cycles without any significant loss in its catalytic activity, thus rendering this precious metal/acid dual-functional catalyst a potential candidate for efficient GVL production under mild conditions.


 Please wait while we load your content...
Please wait while we load your content...