A microscopic and spectroscopic study of rapid antimonite sequestration by a poorly crystalline phyllomanganate: differences from passivated arsenite oxidation†
Abstract
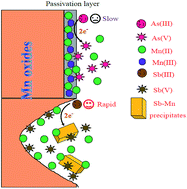
During the reaction of arsenite (As(III)) with δ-MnO2 (a typically poorly crystalline phyllomanganate), a significant decrease in the oxidation rate is frequently observed, which is mainly attributed to the surface passivation of δ-MnO2. However, whether surface passivation also occurs during the antimonite (Sb(III)) oxidation process is unclear. In this study, the behavior and mechanisms of Sb(III) oxidation were compared with those of As(III) during their reactions with δ-MnO2. The experimental kinetics results indicated that the oxidation rate of Sb(III) was 6.14–44.71 times faster than that of As(III) with initial concentrations ranging from 100 to 1000 μM. The macroscopic and spectroscopic results suggested that surface passivation during the adsorption of Mn(II) and the formation of Mn(III) were the predominant causes for the decrease in the As(III) oxidation rate, whereas surface passivation may not have been the limiting factor during Sb(III) oxidation. Compared to As(III) oxidation, the rapid oxidation of Sb(III) by δ-MnO2 led to significant changes in the structure and properties of δ-MnO2, and contributed to the precipitation of Mn(II) antimonate (MnSb2O6). The results of this study facilitate a better understanding of the environmental behavior of Sb and As on metal-oxide surfaces in aquatic environments.


 Please wait while we load your content...
Please wait while we load your content...