Propionic acid-based deep eutectic solvents: synthesis and ultra-deep oxidative desulfurization activity
Abstract
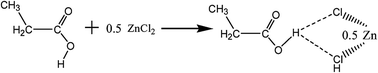
Propionic acid-based deep eutectic solvents (C3H6O2/X ZnCl2, X from 0.1 to 0.6) were synthesized by stirring a mixture of propionic acid and zinc chloride at 100 °C. C3H6O2/0.5 ZnCl2 was characterized by infrared spectroscopy, electrospray ionization mass spectrometry and 1H NMR spectroscopy. The oxidative desulfurization (ODS) of model oil and gasoline was investigated with C3H6O2/0.5 ZnCl2 DESs as an extractant and catalyst, and hydrogen peroxide (H2O2) as an oxidant. Some influence factors such as acidity of DESs, reaction temperature, H2O2 to sulfur (H2O2/S) molar ratio, and volume ratio of DESs to oil were studied. The results indicated that the desulfurization rates of dibenzothiophene (DBT), 4,6-dimethyl-dibenzothiophene (4,6-DMDBT), and gasoline can reach 99.42%, 98.80%, and 66.67%, respectively, under conditions of 30 °C, an H2O2/S molar ratio of 4, and volume ratio of DESs to oil of 0.15/1 over a period of 180 min. The desulfurization rate of DBT in model oil reached 96.31% after five recycles in the C3H6O2/0.5 ZnCl2–H2O2 systems.


 Please wait while we load your content...
Please wait while we load your content...