Influence of molecular interplay on the HPAM/UR rheological properties in an aqueous solution
Abstract
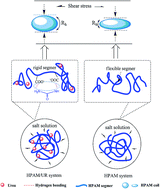
Herein, the interaction between partially hydrolyzed polyacrylamide (HPAM) and urea (UR) in an aqueous solution was characterized via differential scanning calorimetry (DSC) and two-dimension correlation spectra (2DCOS). The rheological properties of the HPAM solution (2000 and 2500 mg L−1) were studied as a function of the addition of the UR content (50, 100, 200, 300, and 400 mg L−1). The results indicate that the HPAM/UR solution exhibits higher intrinsic viscosity, apparent viscosity, first normal stress difference, and modulus than the HPAM solution due to the larger hydraulic size of HPAM in the aqueous solution. From the DSC and 2DCOS results, a stronger hydrogen bonding interaction between carbonyl and amino groups was observed in the vicinity of 45 °C. Dynamic light scattering (DLS) was employed to directly analyze the hydrodynamic diameter, and it further confirmed that a certain amount of UR enabled the blending solution to increase the hydraulic size. Moreover, an interplay model was put forward based on the rheology and dynamic light scattering data.


 Please wait while we load your content...
Please wait while we load your content...