An experimental study on the effects of freeze–thaw cycles on phosphorus adsorption–desorption processes in brown soil
Abstract
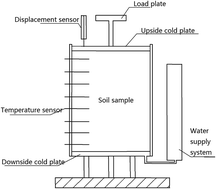
Freeze–thaw cycles (FTCs) can strongly influence the physical and chemical properties of soils in cold regions, which can in turn affect the adsorption–desorption characteristics of phosphorus (P) in the soil. In this study, a series of one-dimensional laboratory freeze–thaw experiments were conducted to determine the adsorption–desorption behavior of P in soil. Results showed that greater numbers of FTCs were associated with a decrease in the adsorption capacity of P in soil. Langmuir and Freundlich equations were adopted to fit the isothermal adsorption curves of P in soil. Values of the critical fitting constant indicated that FTCs weakened the absorption capacity of P in soil. At identical concentrations of exogenous P, the P desorption ratio for soil samples treated with FTCs was greater compared with the ratio measured in untreated soil.


 Please wait while we load your content...
Please wait while we load your content...