Synthesis of phosphonic acid ring-substituted polyanilines via direct phosphonation to polymer main chains†
Abstract
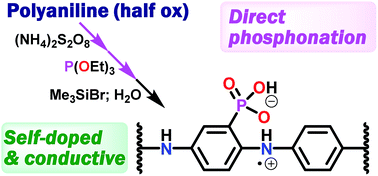
Phosphonic acid ring-substituted polyanilines (PhosPANIs) were synthesized via reductive phosphonation of pernigraniline (a fully oxidized form of PANI) with P(OEt)3 and subsequent hydrolysis. The carbon–phosphorus bond formation was suggested from 31P NMR. This is the first example of direct phosphonation of PANI. The formal substitution ratio of the phosphonate to total number of phenyl rings in the polymer after the phosphonation reaction was up to 52%. The repetition of the phosphonation reaction procedure gave the diethyl PhosPANI (EtPhosPANI) with 73% substitution ratio. Hydrolysis of EtPhosPANIs afforded the desired PhosPANIs. The self-doping of the obtained PhosPANIs was clearly exhibited by ESR, XPS and UV-vis-NIR absorption spectroscopy. The drop-cast films of PhosPANIs showed electrical conductive properties with a level suitable for charge dissipation materials. The developed synthetic approach is considered to be valuable as a practical method for phosphonic acid self-doped conductive PANIs.


 Please wait while we load your content...
Please wait while we load your content...