Synthesis and characterization of some nanostructured composite oxides for low temperature catalytic combustion of dilute propane
Abstract
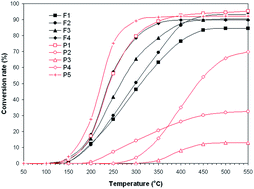
Powders of five nanosized perovskites, SrMnO3, SrCoO3, GdAlO3, FeMnO3, La0.6Pb0.2Mg0.2MnO3, and four ferrospinels, MgFe2O4, Ni0.5Co0.5Fe2O4, Ni0.5Co0.5Fe1.9Sc0.1O4, Ni0.5Co0.5Fe1.8Sc0.2O4, were prepared by a sol–gel self-combustion technique. The powders were catalytically tested in the flameless combustion of dilute propane at atmospheric pressure. All samples had nanocrystalline structures and the crystallite sizes were in the range of 26–89 nm. Catalytic testing showed a significant dependence of the catalytic performance on the catalyst composition. As experiments revealed, the La0.6Pb0.2Mg0.2MnO3 perovskite catalyst, with a specific surface area of 8.6 m2 g−1, exhibits the best performance in propane conversion, of about 92% at 350 °C. The lowest catalytic efficiency was obtained for FeMnO3 and SrCoO3 catalysts. Different reactivities of active oxygen species involved in the catalytic oxidation reaction determine registered differences in catalytic activity of oxide compounds. The results suggest that the La0.6Pb0.2Mg0.2MnO3 perovskite can be a suitable catalyst for catalytic combustion of dilute propane at low temperatures (below 400 °C).


 Please wait while we load your content...
Please wait while we load your content...