(−)-Epigallocatechin-3-gallate (EGCG) inhibits fibrillation, disaggregates amyloid fibrils of α-synuclein, and protects PC12 cells against α-synuclein-induced toxicity
Abstract
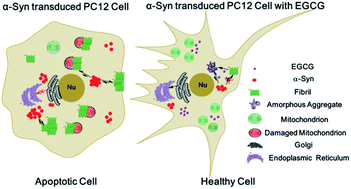
α-Synuclein (α-Syn) aggregates are the major component of Lewy bodies (LB), which is a pathological hallmark in the brain tissue of Parkinson's disease (PD) patients. It has been reported that (−)-epigallocatechin-3-gallate (EGCG) is biologically able to penetrate the blood–brain barrier and inhibit the fibrillation of amyloid proteins. This study aimed to provide insight into the possible mechanism of EGCG as a potential candidate agent for the prevention and treatment of PD on the basis of the interaction between α-Syn and EGCG. In the present study, the effects of EGCG on the fibrillation and disaggregation of α-Syn were investigated by thioflavin T (ThT) fluorescence spectroscopy, circular dichroism spectroscopy (CD), nuclear magnetic resonance (NMR) spectroscopy, atomic force microscopy (AFM) and transmission electron microscopy (TEM) on a molecular level. In addition, on the cellular level, we investigated the protective effects of EGCG on α-Syn-induced cell death in the transduced PC12 cells which overexpressed α-Syn, using the techniques of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) assay, western blot and confocal laser scanning microscopy. It was found that EGCG not only significantly inhibited the conformational transition of α-Syn from random coil to β-sheet conformers through binding to Ile, Phe and Tyr amino residues, but also disaggregated the amyloid fibrils of α-Syn in a dose-dependent manner, through binding to Leu, His, Phe and Tyr amino residues. It is also demonstrated that EGCG can protect PC12 cells against α-Syn-induced damage by inhibiting the overexpression and fibrillation of α-Syn in the cells.


 Please wait while we load your content...
Please wait while we load your content...