The growth mechanism of calcareous deposits under various hydrostatic pressures during the cathodic protection of carbon steel in seawater
Abstract
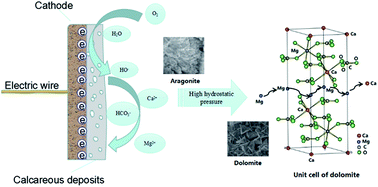
A galvanostatic method was used to study the influence of hydrostatic pressure on the cathodic protection of carbon steel in seawater. The polarization and electrochemical behavior of Q235 carbon steel was studied after being covered with calcareous deposits. Surface analysis was performed to investigate the growth mechanism of the calcareous deposits under various hydrostatic pressures. Although there was no obvious effect of the hydrostatic pressure on the polarization potential decay, the protectiveness of calcareous deposits showed significant variation under the different pressures. The results of electrochemical impedance spectroscopy (EIS) and linear polarization resistance (LPR) indicate that the electrochemical reaction resistance Rct on the polarized metal surface initially increased with the increasing pressure and then decreased, with a maximum value at 2 MPa. The protection factor Fp of the deposits decreased when the pressure was higher than 2 MPa. Scanning electron microscopy (SEM) and X-ray diffraction (XRD) analyses illustrated that parts of the calcium lattice positions were substituted by magnesium ions in the initial crystallization of calcium carbonate under 5 MPa pressure or even higher, which resulted in a crystal structure transformation from aragonite to dolomite. The order degree δ of dolomite decreased under high pressure due to the low free energy of the lattice.


 Please wait while we load your content...
Please wait while we load your content...