Base catalysed N-functionalisation of boroxazolidones†
Abstract
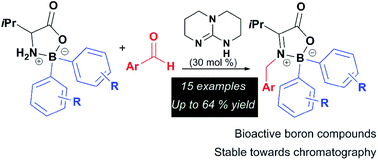
A method for the condensation of boroxazolidones derived from L-valine with aromatic aldehydes, catalysed by 1,5,7-triazabicyclo[4.4.0]dec-5-ene was developed. The preparation and isolation of a series of highly functionalised stable ketimines derived from the reaction of 2,2-diaryl-1,3,2-oxazaborolidin-5-ones with aryl aldehydes is herein described. Several unreported boroxazolidones were prepared by condensation of triethylammonium tetra-arylborates with L-valine in up to 98% yield. The newly synthesised compounds were determined to be moderately cytotoxic against colorectal adenocarcinoma cells, with the best compound in this series having an IC50 of 76 μM. A brief inspection of the effect of the same compound against human brain astrocytoma cells showed an IC50 of 268 μM.


 Please wait while we load your content...
Please wait while we load your content...