Exploring the pathways of aromatic carboxylic acids in ozone solutions
Abstract
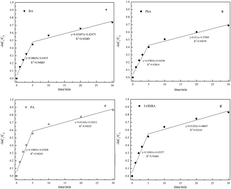
The reaction between ozone and natural organic matter (NOM) generates a certain amount of aromatic carboxylic acids (ACAs). Previous studies report that the pre-ozonation of fulvic acid can produce protocatechuic acid (PA), 3-hydroxybenzoic acid (3-OHBA), phthalic acid (PhA), and benzoic acid (BA). Ozonation of ACAs may result in diverse, low-molecular-weight carbonyl compounds, significantly affecting the formation of disinfection by-products (DBPs) following chlorination. The purpose of this study was to determine the degradation mechanisms, possible ozonation reaction pathways, ozonation intermediates and DBPs of four ACAs, and evaluate the ability of degrading micro-pollutants with different structures by ozonation, as well as to make up for the knowledge gap of DBPs generated. The results showed that two stages pseudo-first-order reaction for four ACAs and six intermediates accompanied the ozonation of ACAs, namely formaldehyde, glyoxal, methylglyoxal, fumaric acid, formic acid, and acetic acid. Whether the hydroxyl radical was involved or not, the ozonation of ACAs contained three steps; first step being the BA hydroxylation. Second, hydroxylation products of BA were oxidized to generate ring-opened compounds such as unsaturated carbonyl compounds. Third, short-chain aldehydes and carboxylic acids were formed. As for mineralization, the short-chain aldehydes and carboxylic acids were finally transformed into CO2 and H2O. In addition, not only ACAs themselves were the main DBPs precursors, but the intermediates of ACAs ozonation could also be the DBPs precursors, and they produced high yields of trihalomethanes (THMs) and haloacetic acids (HAAs). Therefore, the ACA levels should be kept under control in drinking water treatment plants.


 Please wait while we load your content...
Please wait while we load your content...