Self-template synthesis of ATiO3 (A = Ba, Pb and Sr) perovskites for photocatalytic removal of NO†
Abstract
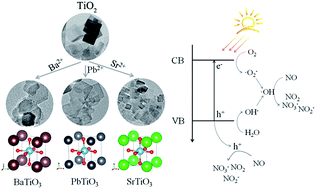
In this study, we report a self-template hydrothermal method for the synthesis of ATiO3 (A = Ba, Pb, and Sr) perovskites using anatase TiO2 nanosheets as precursors. Under hydrothermal conditions, ATiO3 with different structures and morphology can be obtained. These samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) techniques. The growth mechanism of ATiO3 was explored by XRD evaluation over different reaction time, and it was observed that PbTiO3 grows most slowly among all the samples including BaTiO3, SrTiO3, BaxPb1−xTiO3 and SrxPb1−xTiO3. The diffusion of A site sources to the solid surface and the reaction at solid/liquid interface could dominate the growth of ATiO3. All the as-prepared samples exhibited activities toward photocatalytic oxidation of NO at ppb level. Specifically, PbTiO3 has revealed the highest activity under both full spectrum and visible-light irradiation (λ > 420 nm), whereas BaTiO3 exhibited best selectivity for the formation of ionic species (NO3−), which could be ascribed to different electronic structures and charge separation efficiency of ATiO3. This study provides some important hints to tune the photocatalytic performances (activity and selectivity) of ATiO3 through the modification of A site elements.


 Please wait while we load your content...
Please wait while we load your content...