Iminoboronate-based dual-responsive micelles via subcomponent self-assembly for hydrophilic 1,2-diol-containing drug delivery†
Abstract
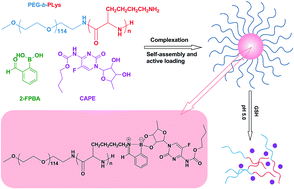
Amphiphilic block copolymer micelles in aqueous solution have been used extensively in delivery of hydrophobic drugs with the hydrophobic core serving as the reservoir. However, encapsulation of hydrophilic drugs by polymeric micelles with hydrophobic core is relatively difficult because of the poor interaction between them. Herein, we report a novel kind of pH/GSH dual-responsive complex micelles with a hydrophilic drug loaded, via direct subcomponent self-assembly of block copolymer poly(ethyleneglycol)-block-poly(L-lysine) (PEG-b-PLys), 2-formylbenzeneboronic acid (2-FPBA) and hydrophilic 1,2-diol-containing drugs (e.g. capecitabine (CAPE)) under physiological pH 7.4 based on the synergistic formation of an iminoboronate structure. The PEG-b-PLys/2-FPBA micelles without CAPE are well-characterized in terms of micellization mechanism, size, morphology, pH- and GSH-responsiveness. Encapsulation of CAPE by forming PEG-b-PLys/2-FPBA/CAPE complex micelles via synergistic formation of the iminoboronate structure is discussed and pH- and GSH-responsive drug-release studies are carried out. The complex micelles are stable under physiological neutral conditions but could be destroyed in response to the stimuli of physiological acidic condition (pH 5.0) and/or glutathione (GSH) at pH 7.4. pH- and GSH-responsive drug-releases are successfully achieved. The drug-loaded complex micelles could be endocytosed by HepG2 cells and efficiently deliver hydrophilic drugs into them. This type of complex micelles could be used as a promising platform for the delivery of hydrophilic 1,2-diol-containing drugs.


 Please wait while we load your content...
Please wait while we load your content...