Biodegradation of the neonicotinoid insecticide acetamiprid in surface water by the bacterium Variovorax boronicumulans CGMCC 4969 and its enzymatic mechanism†
Abstract
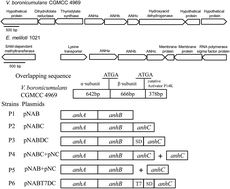
The plant growth-promoting rhizobacterium Variovorax boronicumulans CGMCC 4969 was used to degrade the neonicotinoid insecticide, acetamiprid (AAP), in surface water, and the enzymatic mechanisms of AAP degradation in V. boronicumulans CGMCC 4969 were explored. V. boronicumulans CGMCC 4969 degraded 34.7% of 2 mg L−1 AAP over 120 h with a degradation half-life of 182 h in surface water, and the major metabolite was the amide product, (E)-N2-carbamoyl-N1-[(6-chloro-3-pridyl) methyl]-N1-methylacetamidine (IM-1-2). Gene cloning and over-expression studies proved that AAP hydration to IM-1-2 was mediated by a nitrile hydratase (ANHase). Addition of AAP to the mineral salt medium (MSM) broth significantly upregulated the ANHase gene expression by 1.6-fold, when compared with that in the control without AAP. Co-expression of the ANHase gene with its activator gene (anhC) apparently increased ANHase activity 21-fold for AAP hydration compared with the ANHase gene alone. The independent over-expression of anhC gave rise to competitive inhibition on the β-subunit of the ANHase and resulted in decreased ANHase activity. This ANHase is versatile, hydrating aromatic, N-heterocyclic, and aliphatic nitrile compounds. The present study shows the potential of V. boronicumulans CGMCC 4969 in the bioremediation of AAP contaminated water.


 Please wait while we load your content...
Please wait while we load your content...