Solvothermal synthesis of LiFe1/3Mn1/3Co1/3PO4 solid solution as lithium storage cathode materials
Abstract
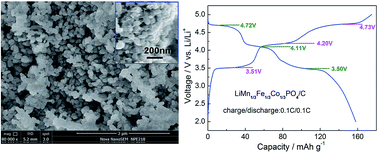
Well-dispersed LiFe1/3Mn1/3Co1/3PO4 nanoparticles with dimensions of ca. 90–180 nm are synthesized by a solvothermal technique using water–ethylene glycol mixture as reaction media. The as-prepared LiFe1/3Mn1/3Co1/3PO4 nanoparticles are well crystallized in the orthorhombic olivine structure and exist in the form of a single-phase solid solution. In LiFe1/3Mn1/3Co1/3PO4 solid solution, the Fe3+/Fe2+and Co3+/Co2+ redox couples exhibit superior electrochemical activity and reversibility compared to the Mn3+/Mn2+ redox couple. Benefiting from the integrated carbon coating and contiguous carbon network, the LiFe1/3Mn1/3Co1/3PO4/C composite delivers a high discharge capacity of 165 mA h g−1 at 0.05C, 148 mA h g−1 at 0.5C, and 120 mA h g−1 at 5C. Meanwhile, it shows considerable cycling stability after 100 cycles at both 25 °C (80% capacity retention at 0.1C) and 50 °C (79% capacity retention at 1C).


 Please wait while we load your content...
Please wait while we load your content...