Insights into the one-electron reduction behavior of tetrachloro-o-benzoquinone: a DFT and molecular dynamics study
Abstract
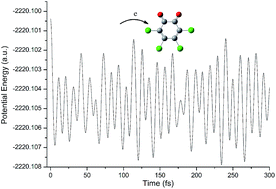
In this study, the one-electron reduction behavior of tetrachloro-o-benzoquinone (o-TCBQ) has been systematically investigated at the B3LYP/6-311++G** level of theory in combination with the ab initio molecular dynamics. It was found that the explicit water molecules have slight effects on the geometry of o-TCBQ. On the contrary, the introduction of an electron can make the geometry change significantly. Moreover, the C2v symmetry of neutral and anionic o-TCBQ in the gas phase has been changed to be C2 symmetry in solution. All the electron affinity and vertical detachment energies are positive in the gas phase and in solution, increasing with the increasing of the dielectric constant of the bulk solvent. Therefore, explicit water molecules and bulk solvents can efficiently enhance the electron-accepting ability of the o-TCBQ, reflecting the intrinsic nature of o-TCBQ as a good electron acceptor in different media.


 Please wait while we load your content...
Please wait while we load your content...