High conversion of glucose to 5-hydroxymethylfurfural using hydrochloric acid as a catalyst and sodium chloride as a promoter in a water/γ-valerolactone system
Abstract
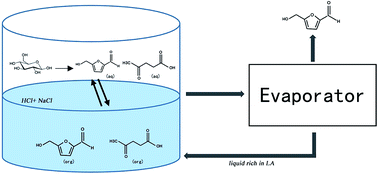
Biomass derived 5-hydroxymethylfurfural (HMF) is regarded as an important platform molecule for the synthesis of value-added chemicals and fuels, but the high production cost has always been a bottleneck for the industrial scale use of HMF. Different mineral acids (HCl and H2SO4) being used as the catalyst and different salts being used as the reaction promoter were evaluated. It was found that HCl, in combination with NaCl, in a water/γ-valerolactone system showed high selectivity and impressive efficiency for the synthesis of HMF from glucose. The optimal conditions to obtain the best HMF yield (62.45%) were 0.2 M HCl and 0.1 M NaCl at 140 °C with a residence time of 60 minutes. An 18.22% molar yield of LA was obtained as a by-product. The effect of different anions was also investigated, and it was determined that not only the hydrogen ions, but also the nature of the acid and the type of salt played a joint role in improving the HMF yield. In addition, a possible synthesis pathway was proposed for large scale production of HMF.


 Please wait while we load your content...
Please wait while we load your content...