A novel modified MIL-101-NH2 ligand for CuI-catalyzed and air promoted oxidation of secondary alcohols†
Abstract
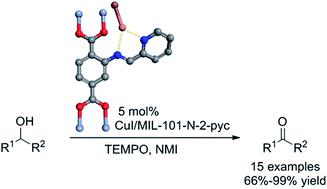
An efficient Cu(I)-catalyzed aerobic alcohol oxidation system was developed utilizing a novel metal–organic framework (MOF) ligand at room temperature. Several relatively inert secondary alcohols were converted to their corresponding ketones in high yields and selectivities in the presence of a Cu(I) catalyst and air as the oxidant. This newly developed Cu(I)/MOF ligand system can also be easily extended to the aerobic oxidation of primary alcohols including aliphatic ones. Furthermore, the MIL-101-N-2-pyc ligand can be recycled several times without compromising reaction activity.


 Please wait while we load your content...
Please wait while we load your content...