Organocatalytic asymmetric conjugate addition of t-butyl nitroacetate to o-quinone methides: synthesis of optically active α-nitro-β,β-diaryl-propionates†
Abstract
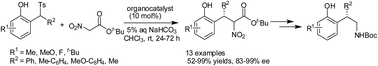
An asymmetric conjugate addition of t-butyl nitroacetate to in situ generated o-quinone methides had been developed. A chiral squamide derived from 9-amino-9-deoxyepiquinine was found to be the efficient catalyst. α-Nitro-β,β-diaryl-propionates could be obtained in good yields and with excellent enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...