Molecular docking, molecular dynamics simulation, biological evaluation and 2D QSAR analysis of flavonoids from Syzygium alternifolium as potent anti-Helicobacter pylori agents†
Abstract
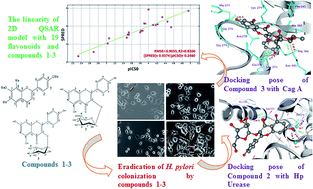
The present study was carried out with the specific aim to evaluate anti-Helicobacter pylori (Hp) and urease inhibitory activities of three flavonoids, namely 5-hydroxy-7,4′-dimethoxy-6,8-di-C-methylflavone (1), kaempferol-3-O-β-D-glucopyranoside (2) and kaempferol-3-O-α-L-rhamnopyranoside (3), of Syzygium alternifolium fruits. These flavonoids were examined for anti-H. pylori activity against two Hp strains, Hp 26695 and Hp P12, through a microbroth dilution assay with a time kill kinetics study and also the evaluation of their ability to lower H. pylori colonization with AGS (gastric epidermal cells). In addition, a urease inhibition assay was performed with these compounds, followed by 2D QSAR, molecular docking and molecular dynamics simulations on these compounds with target proteins, urease and cytotoxin associated gene (Cag A). The in vitro studies showed that compounds 2 and 3 show significant anti-H. pylori activity whereas compound 1 exhibits moderate activity when compared to amoxicillin and that these compounds also show strong bactericidal kinetics in a time dependent manner. These compounds potentially reduce the H. pylori colonization with a significant loss of its adhesion with AGS cells and inhibit the Hp urease activity. 2D QSAR analysis reveals that these compounds exhibit an acceptable correlation of RMSE = 0.905 and R2 = 0.820 with the biological assays. The compounds show strong inhibitions by forming H-bonding interactions with the active pocket residues of the target proteins as evidenced by 10 ns molecular dynamics simulations. Hence, the current investigation will provide a new vision for the discovery of potent antimicrobial agents from natural sources against H. pylori infections.


 Please wait while we load your content...
Please wait while we load your content...