Ionic salt (4-ethoxybenzyl)-triphenylphosphonium bromide as a green corrosion inhibitor on mild steel in acidic medium: experimental and theoretical evaluation
Abstract
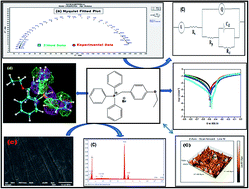
A new phosphonium salt (4-ethoxybenzyl)-triphenylphosphonium bromide (EBTPPB), having different substituents attached to phosphorous and having different anions, is investigated as an inhibitor for mild steel (MS) corrosion in 0.5 M H2SO4 solutions via electrochemical polarization and electrochemical impedance (EI) spectroscopy. Electrochemical results show that EBTPPB compound has practically good inhibiting features for MS corrosion in the corrosive medium with efficiencies of approximately 98% at an optimum 10−2 M concentration. The inhibition is of a mixed cathodic–anodic type. Passive potential (Epp) of the modified steel specimen is in the inactive region and thus inhibits the corrosion process. Langmuir Adsorption (LA) isotherm was performed to provide precise information on the adsorption behavior of the ionic salt. It exhibits both physisorption and predominantly chemisorption mechanism on MS surface. Scanning Electron Microscopy (SEM) associated with Energy Dispersion X-ray (EDX) and Atomic Force Microscopy (AFM) assessment of the electrode surface is consistent with the existence of adsorbing screen of EBTPPB molecules. An apparent connection was ascertained between the experimental corrosion inhibition efficiency (IE%) and the theoretical parameters using quantum chemical calculations.


 Please wait while we load your content...
Please wait while we load your content...