Design, synthesis and biological evaluation of valepotriate derivatives as novel antitumor agents†
Abstract
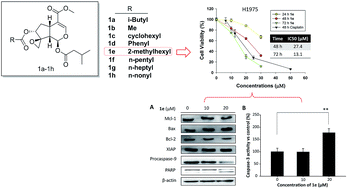
Natural products remain the largest resources of lead compounds that can be used to develop novel anticancer drug candidates. Based on deacetylisovaltratum, a natural product with promising anticancer activity, herein we designed and synthesized of a series of valepotriate derivatives with a novel skeleton from commercially available genipin. In addition, a structure–activity relationship study demonstrated the importance of an epoxy group on the C1-position and the preferable size of the sidechain ((5-methylhexanoyl)oxy) on the C-7 position of valepotriates for their cytotoxic activities. The most potent compound 1e showed moderate to good IC50 values against various cancer cells, ranging from 10.7 to 50.2 μM, which are comparable to that of deacetylisovaltratum. Additionally, we demonstrate that mitochondrion-mediated apoptosis would be its mechanism of action, thus enlightening the further development of novel valepotriate derivatives.


 Please wait while we load your content...
Please wait while we load your content...