Coupling of anhydro-aldose tosylhydrazones with hydroxy compounds and carboxylic acids: a new route for the synthesis of C-β-d-glycopyranosylmethyl ethers and esters†
Abstract
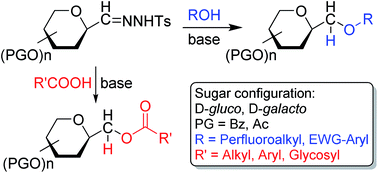
Cross couplings of O-peracylated 2,6-anhydro-aldose tosylhydrazones (C-(β-D-glycopyranosyl)formaldehyde tosylhydrazones) with alcohols, phenols, and carboxylic acids were studied under thermic or photolytic conditions in the presence of K3PO4 or LiOtBu. The reactions failed with EtOH, BnOH, or tBuOH, however, (CF3)2CHOH, electron poor phenols and carboxylic acids gave the corresponding C-β-D-glycopyranosylmethyl ethers and esters, respectively, representing a new access to these glycomimetic compounds.


 Please wait while we load your content...
Please wait while we load your content...