A conceptual model to predict uranium removal from aqueous solutions in water–rock systems associated with low- and intermediate-level radioactive waste disposal†
Abstract
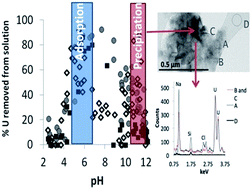
Global stores of radioactive waste are housed in surface stores where actinides are susceptible to environmental release. It is imperative that waste disposal facilities are built to safely contain this waste. However, to do this we must ensure that the engineered and natural barriers are sufficient to prevent the buried materials from migrating through to the surface. Solutions migrating from repositories (ILW and LLW) will have a wide range of chemical compositions and conceptual models constraining the key mineral-water interactions with realistic lithologies are urgently needed. To this end, we conducted experiments to study U removal from solution via mineral-surface interactions with quartz, sandstone, and volcanic rock over a pH range of 2–12, with varying concentrations of U (10 ppb, 0.1 ppm, 1 ppm, and 10 ppm) and with and without bicarbonate added (2 mM) with 0.1 M NaCl electrolyte. We observed that the U concentration in solution had little effect on the extent of U removal from solution as a function of pH or bicarbonate concentration with quartz and sandstone but was important for volcanic rocks, where removal of U, due to adsorption, decreased with increasing U concentration between pH 4 and 8. When bicarbonate was added to solution then the quartz, sandstone, and volcanic rock geomaterials acted similarly in their abilities to immobilize uranium, with an adsorption envelope from pH 4–8 followed by an increase in U removal, likely via precipitation, at high pH. When bicarbonate was not added, the removal of U from solution was more controlled by the geomaterial. Bicarbonate addition at pH 6–10 lowered adsorption. However, the addition of bicarbonate in experiments with 10 ppm U at pH 10–12 allowed for precipitation of U at the rock surface, making bicarbonate an immobilizing factor. Therefore, our conceptual model shows that U is immobilised from radioactive waste-like solutions in a bimodal distribution, both at low (6) and high (11) pH.


 Please wait while we load your content...
Please wait while we load your content...