Direct synthesis of N-sulfinyl- and N-sulfonylimines via copper/l-proline-catalyzed aerobic oxidative cascade reaction of alcohols with sulfinamides or sulfonamides†
Abstract
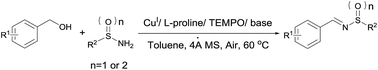
An efficient one-pot synthetic method of N-sulfinyl- and N-sulfonylimines by the condensation of alcohols with sulfinamides or sulfonamides under mild and green conditions has been developed using a combination of CuI, L-proline and TEMPO. This system shows excellent functional group compatibility for a wide range of substrates and affords the corresponding products in good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...