Kinetics based on the base-catalyzed mechanism of a click reaction between glycol dimercaptoacetate and glycidyl phenyl ether†
Abstract
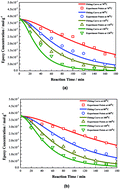
The kinetics model of the thiol–epoxy reaction was built, and the parameters of elementary reactions were obtained according to the well-defined base-catalyzed mechanism. Through the comparison of rate constants, the rate-controlling step was proven to be the deprotonation of thiol by the base catalyst, instead of the nucleophilic attack of thiolate anion on the epoxy functional group, as previously commonly believed. Moreover, alkoxide anions tend to deprotonate unreacted thiol, rather than the conjugate acid of the base catalyst with increasing temperature. Finally, the kinetic model and its parameters have been validated using the experimental data with different initial concentrations of base catalyst, thiol and epoxy functional groups.


 Please wait while we load your content...
Please wait while we load your content...