Electrochemical study on the corrosion behavior of Ti3SiC2 in 3.5% NaCl solution
Abstract
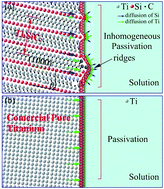
As a newly developed conductive ceramic with wide application prospects, the electrochemical corrosion behavior of Ti3SiC2 in 3.5% NaCl solution was investigated by potentiodynamic polarization, potentiostatic polarization and electrochemical impedance spectroscopy. Commercially pure titanium (CP Ti) was selected for comparative, and cooperated with XRD, XPS, SEM, the passivation behavior, corrosion mechanism were clarified. Ti3SiC2 exhibits typical passivation characteristics and the corrosion resistance at self-corrosion potential is close to that of CP Ti. Silicon and titanium atoms nearby are selectively extracted resulting in a large amount carbon state in the passivation film, which mainly is composed of oxides and hydroxides related to Ti and Si. Lamellar microstructure and the differences between chemical bond of Ti–C and Ti–Si limits the passivation ability, resulting in a weaker passivation ability when compared to that of CP Ti.


 Please wait while we load your content...
Please wait while we load your content...