Immobilization of selenite from aqueous solution by structural ferrous hydroxide complexes†
Abstract
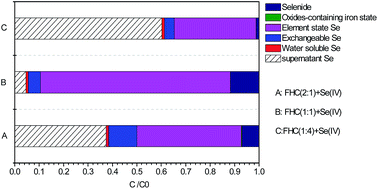
Ferrous hydroxyl complexes (FHCs) were synthesized by precipitation from Fe(II) sulfate salts with sodium hydroxide in an anoxic atmosphere. Effects of the [Fe(II)]/[OH−] ratio, dosage and sequential extraction scheme were elaborated on selenium (Se)(IV) removal by FHCs. Se(IV) removal by FHCs was more coincident with the pseudo-second order kinetic model. The BET isotherm was the best fit model for the data of equilibrium experiments and R2 was 0.9902. Se(IV) removal by FHCs changed with various [Fe(II)]/[OH−] ratios, and the maximum removal capacity was 256.41 mg g−1 by FHC ([Fe(II)]/[OH−] = 1 : 2). X-ray photoelectron spectroscopy results indicated the mechanism of Se(IV) removal by FHC([Fe(II)]/[OH−] = 1 : 1) to be a combination of adsorption and reduction, whereas reduction was the primary mechanism for Se(IV) removal by FHC([Fe(II)]/[OH−] = 1 : 4). A sequential extraction scheme was employed to study Se speciation before and after the reaction. Se species were classified into five groups (water-soluble, exchangeable, elemental Se, oxide-containing iron state, selenide). Results indicated that elemental Se was the primary species in the reaction products.


 Please wait while we load your content...
Please wait while we load your content...